Making Hydrogen Fuel
Today, we depend mostly on gasoline as a fuel for automobiles.
In the future, however, our reliance may shift to a more common
fuel substance, hydrogen. As a clean burning fuel, hydrogen may
be used in new types of internal combustion engines. Of even
greater promise is the use of hydrogen in fuel cells. Fuel cells
harness chemical reactions to produce electric current. Without
the wasteful generation of heat and unwanted pollutants, fuel
cell technology may soon be powering generations of electric
cars.
 * An introduction to the use of hydrogen
as an automobile power source
* An introduction to the use of hydrogen
as an automobile power source
* A hands-on activity in generating hydrogen
* An opportunity to bleach food coloring
Getting Hydrogen
Whether it is to be used as a clean-burning fuel or as a reactant
in fuel cells, sources of hydrogen must be identified. The good
news is that hydrogen is all around, especially in water. Every
molecule of water contains two atoms of hydrogen. Freed from
the water molecule, hydrogen atoms can combine together to form
hydrogen gas. In this activity, you'll generate hydrogen gas
by splitting water in a process called electrolysis.
Materials
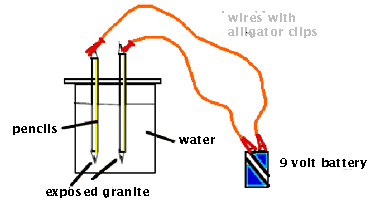
* Connecting wires with alligator clips
at both ends
* 400-mL beaker (or large jar)
* Scrap cardboard
* Two #2 graphite pencils (With graphite exposed at both ends)*
* Tape
* One 9-volt battery
* Salt
* Wax paper
* Food coloring
* Small beaker
* Fumehood (steps 7-10)
CAUTION: If students extend the activity to include steps 7-10, remind them of the dangers of inhaling chlorine gas. These steps should only be performed in a fumehood.
*Teacher note: Prior to the activity, obtain a set of #2 pencils with eraser ends that have been removed. Use a pencil sharpener to expose graphite at both ends of the pencil. Then, dull the pencil points prior to distributing them to students.
Procedure
Basic Prototype
1. Work in teams of two. Cut out a section
of cardboard that is larger than the mouth of the 400-mL beaker.
2. Carefully insert two prepared pencils into side-by-side slots
punched into the cardboard. Make sure the holes are small enough
to hold the pencils tightly in place.
3. Fill the beaker halfway with tap water.
4. Position the cardboard on top of the beaker. Adjust the heights
of the pencils so that the exposed graphite is near the bottom
of the beaker.
5. Use connecting wires to attach the top of each pencil to one
of the 9-volt terminals.
6. Over time, you'll observe gas bubbles collecting on both of
the exposed graphite shafts of the immersed pencils.
7. Fill a small beaker halfway with water and add about 1/2 teaspoon
of table salt.
8. Add a drop of blue food coloring and mix up the solution.
9. Use a dropper to transfer about one mL to this dyed saline
solution to the surface of a sheet of wax paper. Place this sheet
of paper in a fumehood.
10. Within the fumehood, position the exposed pencil tips so
they extend into the liquid. Wait a few moments and you will
observe both the appearance of bubbles and a change in the dye's
intensity. This change in color is caused by the bleaching effect
of generated chlorine.
CAUTION: Do not inhale the generated gas. Chlorine is an irritant.
Questions
1. Consider the polarity of the ions
released when the water decomposed. Which gas collected at the
cathode? Why?
2. Why were there more hydrogen bubbles than oxygen bubbles?
3. Where did the chlorine gas generated in step 10 come from?
Predicton
In addition to generating free chlorine gas, how might adding
a "pinch" of salt affect the decomposition of water?
Redox Model
Consider the process by which water is decomposed into hydrogen
and oxygen gas. Then, compare and contrast it with the fuel cell
process in which these same gases are combined to produce water.
Using toothpicks and gumdrops, construct a representation of
this reversible reaction.
-






- Questions??? For Fast Email Service Email Us Please.
- For FAST Technical Support Please Email Us Now!


 and CHECK OUT
and CHECK OUT
 NO PAPER
company policy. We have no written literature.
NO PAPER
company policy. We have no written literature.
- Over 175,000,000 tons of paper and other wood products are dumped into landfills annually world over.
- We offer only non-polluting electronically generated information that is served here on this web site.
